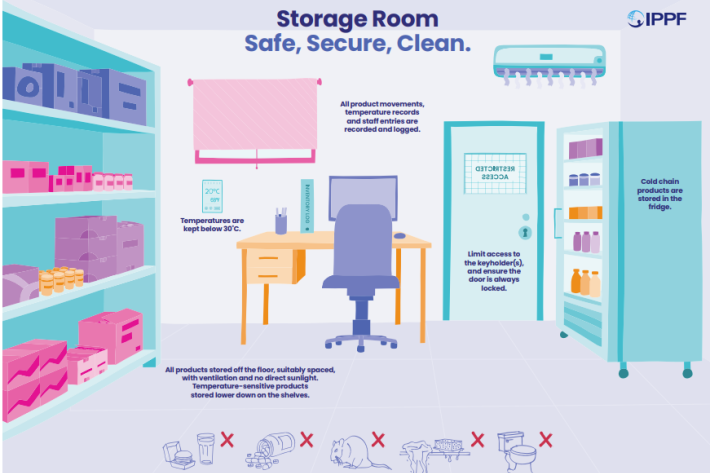
Spotlight
A selection of resources from across the Federation

IMAP Statement on advances in emergency contraception
The purpose of this statement is to review newly published data on increasing the effectiveness of levonorgestrel emergency contraceptive pills by using pre‑coital administration or combined with a non‑steroidal anti‑inflammatory drug; the potential use of LNG‑ECP as a regular contraceptive method for infrequent sex; ulipristal acetate which is an established EC method and is now being studied combined with misoprostol for termination of early pregnancy; and the underutilization of low dose mifepristone as an EC method.
Filter our resources by:

| 09 July 2019
IMAP statement on the ECHO trial
The body of evidence on possible increased risk of HIV acquisition with use of progestogen‑only contraception has remained mixed since 1991, with the greatest concern of an increased risk of HIV acquisition centred on the use of intramuscular depot‑medroxyprogesterone acetate (DMPA‑IM). Data on the risk of HIV acquisition and use of other highly effective contraceptives such as norethisterone enanthate (NET‑EN), hormonal implants, and hormonal and non‑hormonal IUDs are limited.2 And there are no data on subcutaneous DMPA (DMPA‑SC) and HIV risk. In 2016, an updated systematic review of epidemiological evidence on hormonal contraception and HIV acquisition concluded that there was a significant association between the use of DMPA and HIV acquisition and no increased HIV risk with oral contraceptive pills.3 The updated systematic review provided important data regarding DMPA users at high risk of HIV; however, confounding in these observational data could not be excluded. The historically mixed data and the need to control for confounding required further investigation into the association between use of progestogen‑only injectables and increased risk of HIV acquisition, using a more robust research design. This led to the development of the Evidence for Contraceptive Options and HIV Outcomes (ECHO) trial.